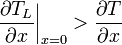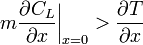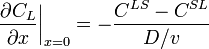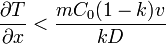Supercooling
Supercooling is the process of chilling a liquid below its melting point, without it becoming solid.
A liquid below its freezing point will crystallize in the presence of a seed crystal or nucleus around which a crystal structure can form. However, lacking any such nucleus, the liquid phase can be maintained all the way down to the temperature at which crystal homogeneous nucleation occurs. The homogeneous nucleation can occur above the glass transition where the system is an amorphous—that is, non-crystalline—solid.
Water has a freezing point of 273.15 K (0 °C or 32 °F) but can be supercooled at standard pressure down to its crystal homogeneous nucleation at almost 231 K (−42 °C).[1] If cooled at a rate on the order of 106 K/s, the crystal nucleation can be avoided and water becomes a glass. Its glass transition temperature is much colder and harder to determine, but studies estimate it at about 165 K (−108 °C).[2] Glassy water can be heated up to approximately 150 K (−123 °C).[1] In the range of temperatures between 231 K (−42 °C) and 150 K (−123 °C) experiments find only crystal ice.
Droplets of supercooled water often exist in stratiform and cumulus clouds. They form into ice when they are struck by the wings of passing airplanes and abruptly crystallize. (This causes problems with lift, so aircraft that are expected to fly in such conditions are equipped with a deicing system.) Freezing rain is also caused by supercooled droplets.
An equivalent to supercooling for the process of melting solids is much more difficult, and a solid will almost always melt at the same temperature for a given pressure. It is, however, possible to superheat a liquid above its boiling point without it becoming gaseous.
Contents |
[edit] Constitutional Supercooling
Constitutional supercooling occurs during solidification, is due to compositional changes, and results in cooling a liquid below the freezing point ahead of the solid-liquid interface. When solidifying a liquid, the interface is often unstable, and the velocity of the solid-liquid interface must be small in order to avoid constitutional supercooling.
Supercooled zones are observed when the liquidus temperature gradient at the interface is larger than the temperature gradient.
or
The slope of the liquidus phase boundary on the phase diagram is 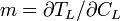
The concentration gradient is related to points, CLS and CSL, on the phase diagram:
The minimum thermal gradient necessary to create a stable solid front is as expressed below.
[edit] See also
[edit] References
- Debenedetti, P. G.; Stanley, H. E. (2003). Supercooled and Glassy Water (PDF). Physics Today 56 (6): 40–46.
- Giovambattista, N.; Angell, C. A.; Sciortino, F.; Stanley, H. E. (July 2004). Glass-Transition Temperature of Water: A Simulation Study (PDF). Physical Review Letters 93 (4).
- Rogerson, M. A.; Cardoso, S. S. S. (April 2004). Solidification in heat packs: III. Metallic trigger. AIChE Journal 49 (2): 522–529.
[edit] External links
- Supercooled Water Matt Sparks's blog entry about his experience with a cold garage and supercooling. Includes videos of supercooled water freezing after being agitated.
- Video example
- Video example #2
| |
Some content on this page may previously have appeared on Citizendium. |