Nucleation
Nucleation is the onset of a phase transition in a small region. The phase transition can be the formation of a bubble or of a crystal from a liquid. Creation of liquid droplets in saturated vapor or the creation of gaseous bubble in a saturated liquid is also characterized by nucleation (see Cloud condensation nuclei). Nucleation of crystalline, amorphous, and even vacancy clusters in solid materials is also important, for example to the semiconductor industry.
Nucleation normally occurs at nucleation sites on surfaces containing the liquid or vapor. Suspended particles or minute bubbles also provide nucleation sites. This is called heterogeneous nucleation. Nucleation without preferential nucleation sites is homogeneous nucleation. Homogeneous nucleation occurs spontaneously and randomly, but it requires superheating or supercooling of the medium. Nucleation is involved in such processes as cloud seeding and in instruments such as the bubble chamber and the cloud chamber.
Contents |
[edit] Examples of nucleation
- Pure water freezes at −42 °C rather than at the melting temperature (0 °C) of ice if no crystal nuclei, such as dust particles, are present to form an ice nucleus.
- Presence of cloud condensation nuclei is important in meteorology because they are often in short supply in the upper atmosphere (see cloud seeding).
- All natural and artificial crystallization process (of formation of solid crystals from a homogeneous solution) starts with a nucleation event.
- Bubbles of carbon dioxide nucleate shortly after the pressure is released from a container of carbonated liquid. Nucleation often occurs more easily at a pre-existing interface (heterogeneous nucleation), as happens on boiling chips and string used to make rock candy. So-called Diet Coke and Mentos eruptions are a dramatic example.
- Nucleation in boiling can occur in the bulk liquid if the pressure is reduced so that the liquid becomes superheated with respect to the pressure-dependent boiling point. More often nucleation occurs on the heating surface, at nucleation sites. Typically, nucleation sites are tiny crevices where free gas-liquid surface is maintained or spots on the heating surface with lower wetting properties. Substantial superheating of a liquid can be achieved after the liquid is de-gassed and if the heating surfaces are clean, smooth and made of materials well wetted by the liquid.
- Nucleation is a key concept in polymer[1], alloy, and ceramic systems.
- In chemistry and biophysics, nucleation can also refer to the phaseless formation of multimers which are intermediates in polymerization processes. This sort of process is believed to be the best model for processes such as crystallization and amyloidogenesis.
- In molecular biology, nucleation is used to term the critical stage in the assembly of a polymeric structure, such as a microtubule, at which a small cluster of monomers aggregates in the correct arrangement to initiate rapid polymerization. For instance, two actin molecules bind weakly, but addition of a third stabilizes the complex. This trimer then adds additional molecules and forms a nucleation site. The nucleation site serves the slow, or lag phase of the polymerization process.as
[edit] Mechanics of nucleation
[edit] Homogeneous nucleation
Nucleation generally occurs with much more difficulty in the interior of a uniform substance, by a process called homogeneous nucleation. Liquids cooled below the maximum heterogeneous nucleation temperature (melting temperature), but which are above the homogeneous nucleation temperature (pure substance freezing temperature) are said to be supercooled. This is useful for making amorphous solids and other metastable structures, but can delay the progress of industrial chemical processes or produce undesirable effects in the context of casting.
The creation of a nucleus implies the formation of an interface at the boundaries of the new phase. Some energy is consumed to form this interface, based on the surface energy of each phase. If a hypothetical nucleus is too small, the energy that would be released by forming its volume is not enough to create its surface, and nucleation does not proceed. The critical nucleus size can be denoted by its radius, and it is when r=r* (or r critical) that the nucleation proceeds.
For example in the classic case[2] of a spherical cluster that liberates -Gv Joules per cubic centimeter during formation (here Gv is a negative quantity), but which must pay the positive cost of σ Joules per square centimeter of surface interfacing with the world around, the free energy needed to form a cluster of radius r is...
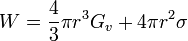 .
.
Graphing this as a function of radius shows that it costs free energy to add molecules to this cluster, until the radius reaches
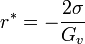 .
.
Addition of new molecules to clusters larger than this critical radius releases, rather than costs, available work. In other words at that point growth of the cluster is no longer limited by nucleation, but perhaps by diffusion[3] (i.e. the supply of molecules) or by reaction kinetics instead.
As the phase transformation becomes more and more favorable, the formation of a given volume of nucleus frees enough energy to form an increasingly large surface, allowing progressively smaller nuclei to become viable. Eventually, thermal activation will provide enough energy to form stable nuclei. These can then grow until thermodynamic equilibrium is restored.
The spontaneous nucleation rate in, say, water changes very rapidly with temperature, so the spontaneous nucleation temperature can be quite well defined. 'Film boiling' on very hot surfaces and the Leidenfrost effect are both believed to be stabilized by spontaneous nucleation phenomena.
[edit] Heterogeneous nucleation
In the case of heterogeneous nucleation, some energy is released by the partial destruction of the previous interface. For example, if a carbon dioxide bubble forms between water and the inside surface of a bottle, the energy inherent in the water-bottle interface is released wherever a layer of gas intervenes, and this energy goes toward the formation of bubble-water and bubble-bottle interfaces. The same effect can cause precipitate particles to form at the grain boundaries of a solid. This can interfere with precipitation strengthening, which relies on homogeneous nucleation to produce a uniform distribution of precipitate particles.
[edit] References
- ↑ R. J. Young (1981) Introduction to Polymers (CRC Press, NY) ISBN 0-412-22170-5
- ↑ F. F. Abraham (1974) Homogeneous nucleation theory (Academic Press, NY)
- ↑ Frank S. Ham (1959) Diffusion-limited growth of precipitate particles, J. Appl. Phys. 30:1518-1525
[edit] External links
- The Extreme Diet Coke & Mentos Experiments - fun with nucleation
| |
Some content on this page may previously have appeared on Citizendium. |

