Dissociation constant
In biochemistry, chemistry and physics, the binding interaction of two molecules that bind with each other, for example a protein and a DNA duplex, is often quantified in terms of a dissociation constant, abbreviated as Kd, which is the inverse of the association constant, or Ka. The strength of the binding interaction is inversely proportional to Kd. Extremely tight-binding molecules such as antibodies and the their target exhibit Kd values in the picomolar range (10−12), while many drugs bind to their targets with Kd values in the nanomolar (10−9) to micromolar (10−6) range. Given the Kd of an interaction, and the initial concentrations of the interacting molecules, the amount of complex can be calculated.
Biomolecular Definition
Given two molecules, A and B, with initial molar concentrations [A]0 and [B]0, that form a reversible binding complex AB, having a certain dissociation constant Kd, that is,
The Kd, by definition, is
Using the facts that [A] = [A]0 − [AB] and [B] = [B]0 − [AB] gives
expanding the top terms yields
Multiplying both sides by [AB] and rearranging gives a quadratic equation:
whose solution is:
Given the physical limitation that [AB] cannot be greater than either [A]0 or [B]0 eliminates the solution in which the square root term is added to the first term.
Implications
An inspection of the resulting solution shown above illustrates that in order to have an appreciable amount of bound material, the interacting molecules must be present at concentrations of 1/100 to 100 times the dissociation constant, as demonstrated in the table below, in which the concentrations of A and B are expressed in units of Kd.
| [A]/Kd | [B]/Kd | %B bound ([AB]/[B])*100 |
|---|---|---|
| 0.001 | 0.001 | 0% |
| 0.01 | 0.01 | 1% |
| 0.1 | 0.1 | 8% |
| 1.0 | 1.0 | 38% |
| 10 | 10 | 73% |
| 100 | 100 | 90% |
| 1000 | 1000 | 97% |
| |
Some content on this page may previously have appeared on Citizendium. |
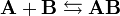
![\mathbf{K_d} = \frac{\mathbf{[A]}\times\mathbf{[B]}}{\mathbf{[AB]}}](images/math/4/7/4/47410195285583fd7759c5feae0486d7.png)
![\mathbf{K_d} = \frac{(\mathbf{[A]_0} - \mathbf{[AB]})\times(\mathbf{[B]_0} - \mathbf{[AB]})}{\mathbf{[AB]}}](images/math/b/a/a/baa698ec8d5c0cb79e23d1b2d3579be3.png)
![\mathbf{K_d} = \frac{\mathbf{[A]_0} \times \mathbf{[B]_0} - \mathbf{[A]_0} \times \mathbf{[AB]} - \mathbf{[B]_0} \times \mathbf{[AB]} + \mathbf{[AB]} \times \mathbf{[AB]}}{\mathbf{[AB]}}](images/math/e/e/2/ee25c520460d76ddfefde8d44f8c91d4.png)
![\mathbf{[AB]^2} - \left(\mathbf{[A]_0} + \mathbf{[B]_0} + \mathbf{K_d}\right) \times \mathbf{[AB]} + \left(\mathbf{[A]_0} \times \mathbf{[B]_0}\right) = \mathbf{0}](images/math/7/d/9/7d9ae03e3f8805a58bdb0ae6d2defba7.png)
![\mathbf{[AB]} = \frac{(\mathbf{[A]_0} + \mathbf{[B]_0} + \mathbf{K_d}) \pm \sqrt{(\mathbf{[A]_0} + \mathbf{[B]_0} + \mathbf{K_d)^2} - \mathbf{4} \mathbf{[A]_0} \mathbf{[B]_0}}}{\mathbf{2}}](images/math/8/3/f/83f0fad58e7ea5b5401696db0dcf6ffc.png)