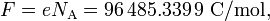Faraday constant
In chemistry and physics, the Faraday constant F is the amount of charge (in absolute value) in one mole of electrons or one mole of monovalent (singly charged) ions. Its value[1] is
where NA is Avogadro's constant and e is the charge of an electron.
The constant F must be carefully distinguished from the unit F (the faraday) which is a unit of capacitance.
The constant and the unit are named after the British physicist Michael Faraday.
Before electrons were discovered and a value for Avogadro's number was known, Faraday discovered (1833) that in electrolysis the amount of charge F necessary to deposit one mole of monovalent ions on an electrode (cations on the cathode, anions on the anode) is always the same, irrespective of the kind of ions. For a long time weighing the amount of silver—which in solution is the cation Ag+—deposited during electrolysis was the accepted manner of measuring electric charge and electric current.
Reference
| |
Some content on this page may previously have appeared on Citizendium. |